For this day's lesson, we had a new professor. He was Sir Tim Bengala. For this day's lesson we started with bond length and strength.Then go through structural isomers, degree of unsaturation, and boiling and melting point.
Bond length is the distance between the nuclei of two bonded atoms and it depends on the size of the bonded atoms. Bond strength is measured by the energy required to break a bond. Bond length and strength are inversely proportional. Stronger bonds tend to be shorter. Double and triple bonds are stronger and shorter than single bonds. But double bonds are not twice as strong as single bonds and triple bonds are not trice as strong as single bonds.
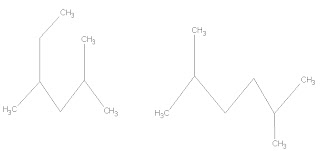
Compounds with different structures and spatial arrangement but same molecular formula are called constitutional isomers. It has different attachment and should not be confused with same compounds that are drawn differently but have the same attachments.
Isomers
Same Compound
The Degree of Unsaturation is the total number of multiple bonds plus rings. The general formula for Degree of Unsaturation is:
The maximum number of hydrogen atoms can be calculated using the equation 2n + 2 wherein n is the number of carbon atoms. Halogens are treated as if they are hydrogens and are added to the actual number of H. For compounds with oxygen, there is no change in formula. If nitogen is present, the total number of nitrogen is added to the maximum possible number of H.
If degree of unsaturation is equal to zero, it means that the compound is saturated and therefore the bonds are all single bond. If degree of unsaturation is equal to one, it means that the compound is unsaturated and therefore have double bond or is cyclic.
Melting point is the range of temperature at which a substance changes from solid to liquid phase. Boiling point corresponds to the temperature at which thermal energy of the molecule is great enough to overcome the cohesive forces that hold them in the liquid state.


Walang komento:
Mag-post ng isang Komento