Our first formal lesson for organic chemistry was a review of atomic and molecular orbitals and chemical bonding. First of all we had a recap of basic chemistry concepts like the atomic structure, quantum numbers, ionic and covalent bonds, and valence-shell electron-pair repulsion (VSEPR). It was good because I can still follow the things he was discussing and understand it but I guess it was because I already know this before hand.
Atomic orbitals is simply the space where electrons spend most of their time. The first orbital is the lowest-energy orbital, the 1s orbital. It has a spherical shape like all s orbitals. It has one math sign all through out the entire orbital, whether it is positive or negative does not affect the charge. The next orbital is 2s orbital. It too has a spherical shape but only larger. It has a region where the value of the wave function is zero. It is called the node.
The next orbital is 2p orbital which has a shape of a dumbbell. It has no center because of the node and has regions of high probability of finding electrons on opposite sides of the nucleus. One region has a positive sign and the other region has negative. There are three 2p orbitals and they are degenerate.
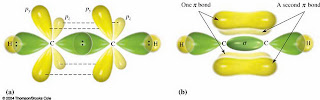
We had discussed molecular orbitals too. When atoms bond some of them share electrons which form molecular orbitals. The shape of molecular orbitals came from the overlap of atomic orbitals. There are two kinds of overlap, the constructive and the destructive. Constructive overlap results to bond formation and the destructive overlap does not. When atomic orbitals overlap, the said overlap happens. Because of this, two kinds of molecular orbital form, the bonding and the antibonding.
We had also discussed the hybridization of methane, ethane, ethene and ethyne which are sp3 hybrids, sp2 hybrids, and sp hybrids and their structure which has a single bond, double bond, and triple bond.
Methane
Ethane
Ethene
Ethyne






Walang komento:
Mag-post ng isang Komento