The various shapes that a molecule can assume by rotation about single bonds are called conformations. The eclipsed conformation has each hydrogen on one carbon as close as possible to one hydrogen on the other carbon. The staggered conformation has the hydrogen on one carbon as far from the hydrogen on the other as possible. Other conformations are intermediate between these two extremes. The staggered conformation is more stable because of the torsional and steric strain in the eclipsed conformation.
Ethane
Butane
Ph Ch 125: Organic Chemistry
Martes, Oktubre 11, 2011
Seventh Meeting: August 1, 2011 - Functional Groups and Nomenclature
Organic compounds are named according to the internationally accepted conventions of IUPAC. This system of nomenclature is summarized as follows:
- The highest priority in the functional groups present should be the suffix name.
- The longest carbon chain provides the stem name. The stem names are derived from the names of hydrocarbons.
- The carbon chain is numbered, keeping minimum values for the suffix group.
- Side-chain substituents are added as prefixes with appropriate numbering, listing them alphabetically.
Sixth Meeting: July 25, 2011 - Cont. Acid and Base; Functional Groups
For this meeting we had a continuation of the Acid and Base topic in the factors affecting the acidity and basicity. After that we discussed briefly the first part of functional groups which was a topic for the second exam.
In naming a compound, the International Union of Pure and Applied Chemistry (IUPAC) provided some guidelines in order for it to be easier to memorize, systematic and organize. It was called the IUPAC nomenclature.
Procedure:
We first discussed Hydrocarbons. Alkane is composed of single bonds and saturated. Alkenes a have double bond and Alkynes have a triple bond. Both of them are unsaturated. The suffix for alkane is -ane. For alkene, it is -ene and for alkyne it is -yne.
Fifth Meeting: July 18, 2011 - Acids and Bases
Most of the reactions in organic chemistry involved proton transfer and so the topic is very useful in our journey through this subject. We discussed the definitions of the following concepts: Arrhenius Concept, Bronsted-Lowry Concept, and Lewis Concept.
Arrhenius Concept is only applicable in an aqueous system. It stated that when in water, acids have hydrogen ions while base have hydronium ions. According to the Bronsted-Lowry Concept, an acid is a proton donor while a base is a proton acceptor. A compound that has both H+ and a pair of electron can act either as an acid or base. It also introduced the concept of conjugate acid-base. It states that an acid is always one unit more positive than its conjugated base while a base is always one unit more negative than conjugate acid. The Lewis Concept states that an acid is an electron pair acceptor while a base is an electron pair donor. All Bronsted-Lowry Base are Lewis Base but not all Lewis Acid are Bronsted-Lowry Acid.
The reaction of an acid with a base is in equilibrium with the conjugate base and conjugate acid products. Atom bonded to acidic hydrogen, hybridization, hydrogen bonding, inductive effect, steric effect, resonance, resonance in aromatic compounds are the factors that affect the acidity and basicity of a compound.
Fourth Meeting: July 11, 2011 - Structural Effects
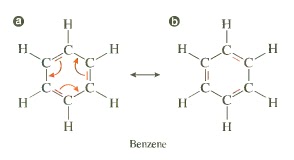
Resonance (Pi Electron Delocalization)
It is a structural effect that can be seen in systems where there are atoms that are sp2 hybridized. Two individual structures of the same compound are called resonance form, their special relationship is indicated by the double-headed arrow between them. The only difference between the resonance forms is the position of the bonding electrons. The atoms themselves remain in exactly the same place in both resonance forms.
Conjugation occurs in any molecule with a series (adjacent) of three or more overlapping p-orbitals.
Resonance Stabilization states that the delocalized molecular orbital is more stable than the localized molecular orbital because of the lower total energy of the delocalized molecular orbital.
Types of Resonance Interaction
1) unshared pair of electrons next to a pi bond
2) pi bonds between atoms of different electronegativity
3) involving a cycle of double bonds
4) involving an empty p orbital
Rules for Resonance Structure
Rule Two: Each resonance structure must have the same number of electrons and the same total charge.
Rule Three: The relative stability of the resonance structure can be judged by the same rules that were previously introduced to judge the stability of Lewis structures - octet rule, location of formal charges, interaction between the charges in the structure.
Rule Four: The actual structure must resemble the most stable resonance structure.
Rule Five: The resonance stabilization energy increases as the number of important or stable structures increases.
Third Meeting: July 4, 2011 - Properties of Organic Compounds
For this day's lesson, we had a new professor. He was Sir Tim Bengala. For this day's lesson we started with bond length and strength.Then go through structural isomers, degree of unsaturation, and boiling and melting point.
Bond length is the distance between the nuclei of two bonded atoms and it depends on the size of the bonded atoms. Bond strength is measured by the energy required to break a bond. Bond length and strength are inversely proportional. Stronger bonds tend to be shorter. Double and triple bonds are stronger and shorter than single bonds. But double bonds are not twice as strong as single bonds and triple bonds are not trice as strong as single bonds.
Compounds with different structures and spatial arrangement but same molecular formula are called constitutional isomers. It has different attachment and should not be confused with same compounds that are drawn differently but have the same attachments.
Isomers
Same Compound
The Degree of Unsaturation is the total number of multiple bonds plus rings. The general formula for Degree of Unsaturation is:
The maximum number of hydrogen atoms can be calculated using the equation 2n + 2 wherein n is the number of carbon atoms. Halogens are treated as if they are hydrogens and are added to the actual number of H. For compounds with oxygen, there is no change in formula. If nitogen is present, the total number of nitrogen is added to the maximum possible number of H.
If degree of unsaturation is equal to zero, it means that the compound is saturated and therefore the bonds are all single bond. If degree of unsaturation is equal to one, it means that the compound is unsaturated and therefore have double bond or is cyclic.
Melting point is the range of temperature at which a substance changes from solid to liquid phase. Boiling point corresponds to the temperature at which thermal energy of the molecule is great enough to overcome the cohesive forces that hold them in the liquid state.
Second Meeting: June 27, 2011 - Everything Starts with Org. Chem.
Our first formal lesson for organic chemistry was a review of atomic and molecular orbitals and chemical bonding. First of all we had a recap of basic chemistry concepts like the atomic structure, quantum numbers, ionic and covalent bonds, and valence-shell electron-pair repulsion (VSEPR). It was good because I can still follow the things he was discussing and understand it but I guess it was because I already know this before hand.
Atomic orbitals is simply the space where electrons spend most of their time. The first orbital is the lowest-energy orbital, the 1s orbital. It has a spherical shape like all s orbitals. It has one math sign all through out the entire orbital, whether it is positive or negative does not affect the charge. The next orbital is 2s orbital. It too has a spherical shape but only larger. It has a region where the value of the wave function is zero. It is called the node.
The next orbital is 2p orbital which has a shape of a dumbbell. It has no center because of the node and has regions of high probability of finding electrons on opposite sides of the nucleus. One region has a positive sign and the other region has negative. There are three 2p orbitals and they are degenerate.
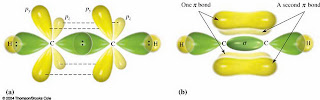
We had discussed molecular orbitals too. When atoms bond some of them share electrons which form molecular orbitals. The shape of molecular orbitals came from the overlap of atomic orbitals. There are two kinds of overlap, the constructive and the destructive. Constructive overlap results to bond formation and the destructive overlap does not. When atomic orbitals overlap, the said overlap happens. Because of this, two kinds of molecular orbital form, the bonding and the antibonding.
We had also discussed the hybridization of methane, ethane, ethene and ethyne which are sp3 hybrids, sp2 hybrids, and sp hybrids and their structure which has a single bond, double bond, and triple bond.
Methane
Ethane
Ethene
Ethyne
Mag-subscribe sa:
Mga Komento (Atom)