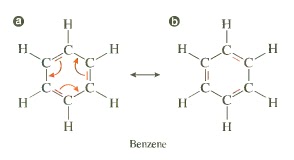
Resonance (Pi Electron Delocalization)
It is a structural effect that can be seen in systems where there are atoms that are sp2 hybridized. Two individual structures of the same compound are called resonance form, their special relationship is indicated by the double-headed arrow between them. The only difference between the resonance forms is the position of the bonding electrons. The atoms themselves remain in exactly the same place in both resonance forms.
Conjugation occurs in any molecule with a series (adjacent) of three or more overlapping p-orbitals.
Resonance Stabilization states that the delocalized molecular orbital is more stable than the localized molecular orbital because of the lower total energy of the delocalized molecular orbital.
Types of Resonance Interaction
1) unshared pair of electrons next to a pi bond
2) pi bonds between atoms of different electronegativity
3) involving a cycle of double bonds
4) involving an empty p orbital
Rules for Resonance Structure
Rule Two: Each resonance structure must have the same number of electrons and the same total charge.
Rule Three: The relative stability of the resonance structure can be judged by the same rules that were previously introduced to judge the stability of Lewis structures - octet rule, location of formal charges, interaction between the charges in the structure.
Rule Four: The actual structure must resemble the most stable resonance structure.
Rule Five: The resonance stabilization energy increases as the number of important or stable structures increases.



Walang komento:
Mag-post ng isang Komento